About the study
The study will provide definitive evidence on the clinical and cost-effectiveness of lithium and quetiapine combination therapy vs lithium vs quetiapine monotherapies in the maintenance treatment of bipolar disorder.

Objectives
Primary objective:
To evaluate whether a combination of lithium and quetiapine increases time to next mood episode (a measure of relapse/symptoms) over 24 months in comparison to lithium or quetiapine in the maintenance treatment of BD.
Secondary objectives:
To evaluate in the maintenance treatment of BD:
- the effectiveness of lithium vs quetiapine on time to new mood episode over 24 months.
- the effectiveness of lithium plus quetiapine vs lithium vs quetiapine on health related quality of life (QoL), functioning, and other important outcomes at 12 and 24 months.
- the cost-effectiveness of lithium plus quetiapine vs lithium vs quetiapine

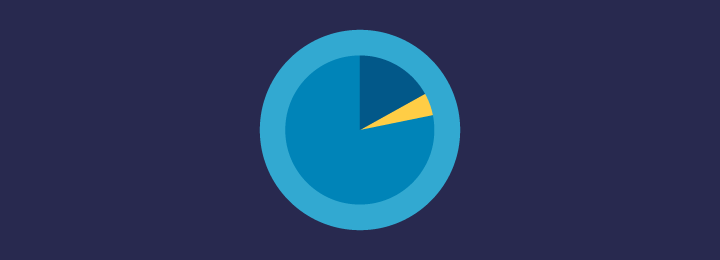
Current enrolment curve
Recruitment target: 303
Locations
Information about the Study

Study Design
A pragmatic, observer-blind, multi-centre, 3-arm RCT comparing lithium plus quetiapine vs lithium vs quetiapine in the maintenance treatment of Bipolar disorder over 24 months.

Treatments
Lithium and quetiapine together, lithium alone, or quetiapine alone.

Study Funders
The study is funded by the National Institute of Health and Care Research (NIHR). The sponsor is the University of Birmingham.
Participant Data
303 adults with bipolar disorder who need maintenance treatment from secondary mental health services, like community mental health teams and early intervention services. Whether participants are asked to take the treatments together or either medication alone will be decided by random chance.

Outcome Measures
Measuring the time to participants becoming ill and having a new high or low mood episode over a two-year period. We will also look at how the treatment effects quality of life, side effects, general impact on health, and coping with everyday tasks. Taking all this information together, we also want to work out which option is better value for money for the NHS.

Things to Know
Researchers will assess participants regularly over two years to see if they have had a new mood episode, or impacts to their quality of life, functioning, what side effects they have had, and use of health services. Other than this, the patient's care will not change. The study will take 68 months to complete. There are two researchers with lived experience of Bipolar disorder on the team who have influenced its design at each stage.
Study Team

Professor Steven Marwaha
Chief Investigator
Steven Marwaha is an academic Psychiatrist whose main interest is in early intervention and mood disorders, such as bipolar disorder and depression. His work focusses on pathways to illness and care, and ways of improving the clinical outcomes of this group using both pharmacological and psychological interventions. As a Consultant Psychiatrist he runs the Specialist Mood Disorders Clinic in Birmingham and Solihull Mental Health Trust.

Sam Hopkins
Senior Trial Manager
Sam is a senior trial manager at Birmingham Clinical Trials Unit based at the University of Birmingham. She has over 13 years of experience working on clinical trials, in a range of different specialities in both an NHS and academic setting.
Altus Chan
Senior Data Manager
Altus is a Senior Data Manager at Birmingham Clinical Trials Unit based at the University of Birmingham. He has been with the Unit for 4 years, with experience in colorectal, women's health, and mental health clinical trials.